• Flexibility in baseline profiling – tissue or liquid biopsy
• Ultra-sensitive MRD detection – 0.005% LOD
• Tracking up to 50 personalized mutations, including fusions
• Detecting 500 actionable mutations
• Including genome-wide copy number changes
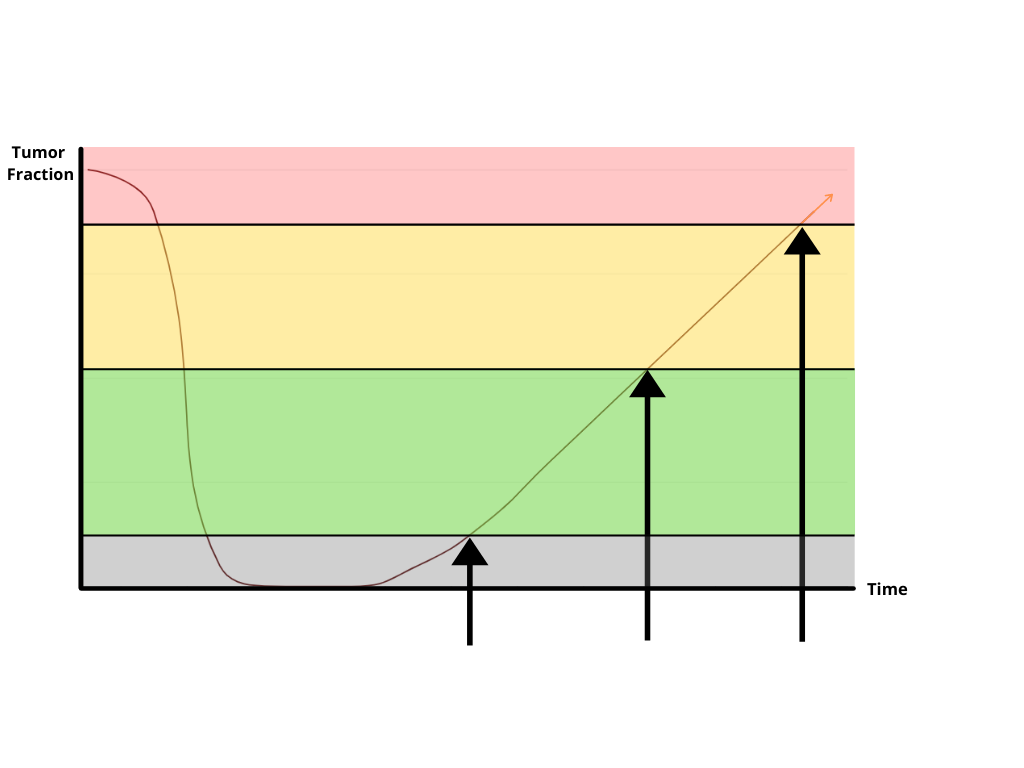




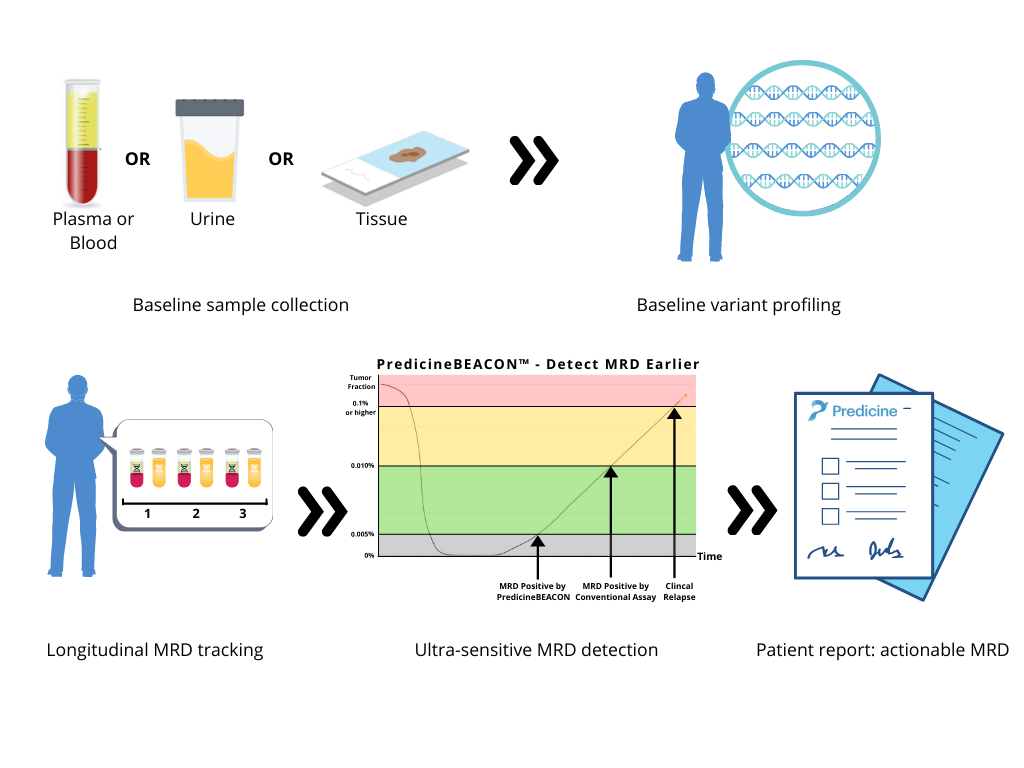
PredicineBEACON™ is designed for ultra-sensitive MRD detection to address the following clinical questions beyond the capacity of conventional MRD assays:
MRD detection with PredicineBEACON™ is different from conventional MRD assays because it is not limited by baseline sample availability. If available, baseline samples can be assessed using patient’s blood, urine or tissue. If a baseline sample is unavailable, monitoring the recurrence or progression of cancer can be achieved with a simple blood draw or urine sample with our tissue-agnostic approach.
We offer pilot program grants to select biopharma and academic partners to empower translational research and clinical studies. To initiate a study, contact us via the form below.
If you have any questions or need assistance, complete this form and we will respond within 24 hours.